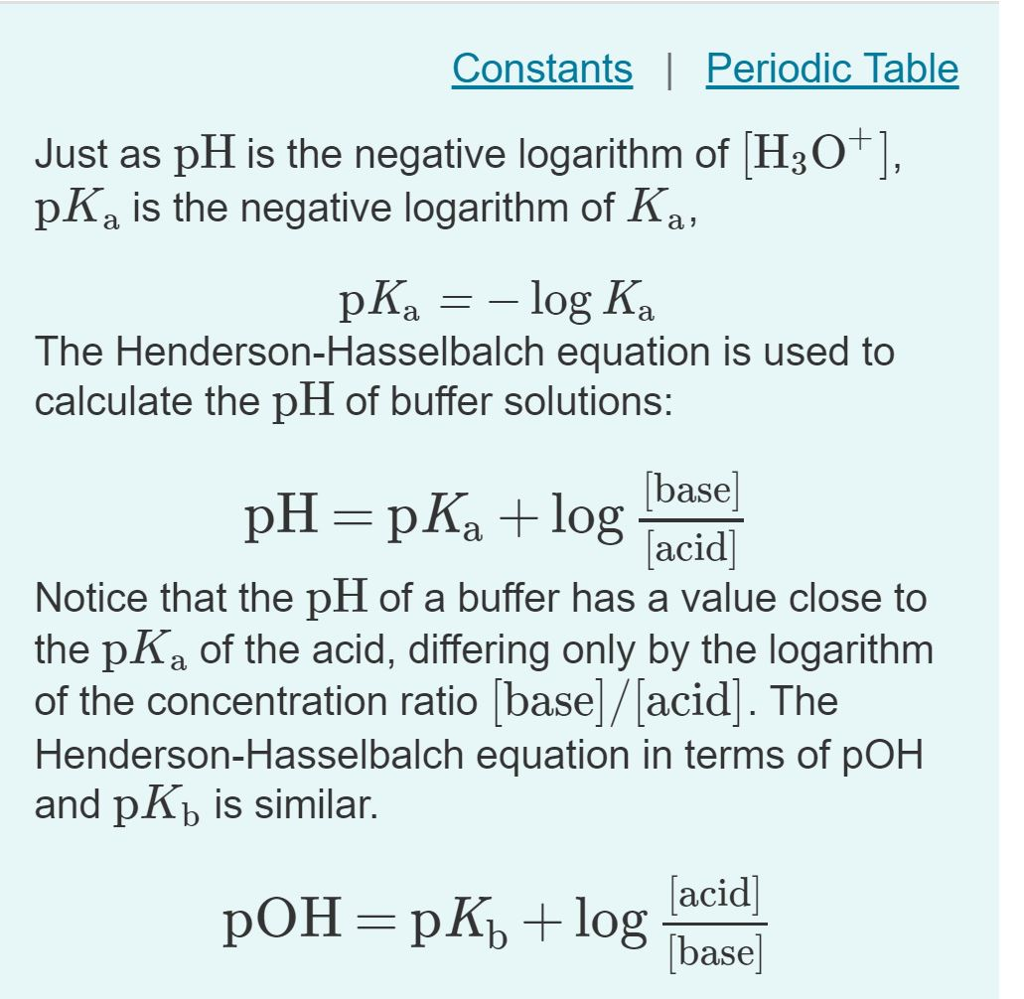
pKa & pH Values| Functional Groups, Acidity & Base Structures - Video & Lesson Transcript | Study.com
![Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube](https://i.ytimg.com/vi/SLPu7qlUdEA/maxresdefault.jpg)
Henderson Hasselbalch Equation Acid Base Buffer Chemistry Introduction ph = pka + log [A/HA] - YouTube
![SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use](https://cdn.numerade.com/ask_images/08a0de4c43b94a8e8f10c82ca0bf2b68.jpg)
SOLVED: What is the pH of a buffer composed of 0.10 M oxalic acid and 0.08 M oxalate ion? The pKa for oxalic acid is 1.23. pH = pKa log ([A-J[HA]) Use
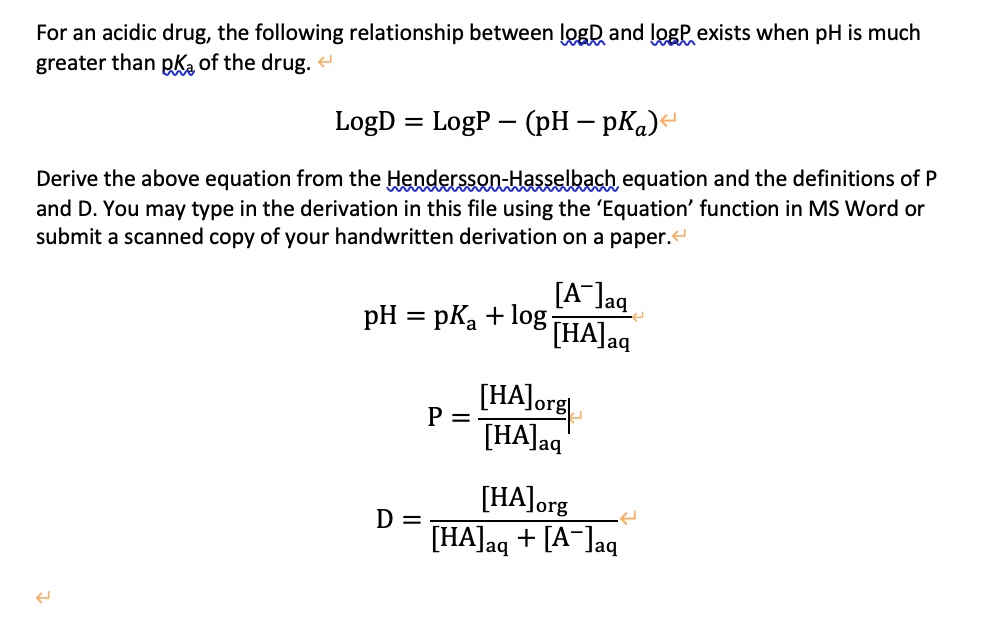
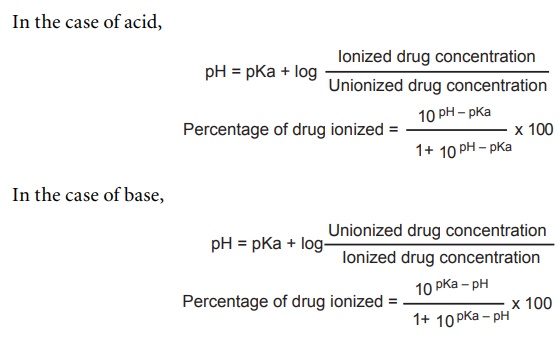
SOLVED: For an acidic drug, the following relationship between logD and lggE exists when pH is much greater than pKa of the drug: LogD = LogP (pH pKa) Derive the above equation
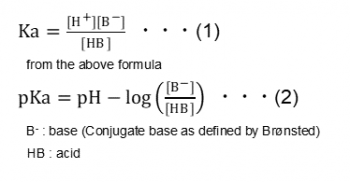


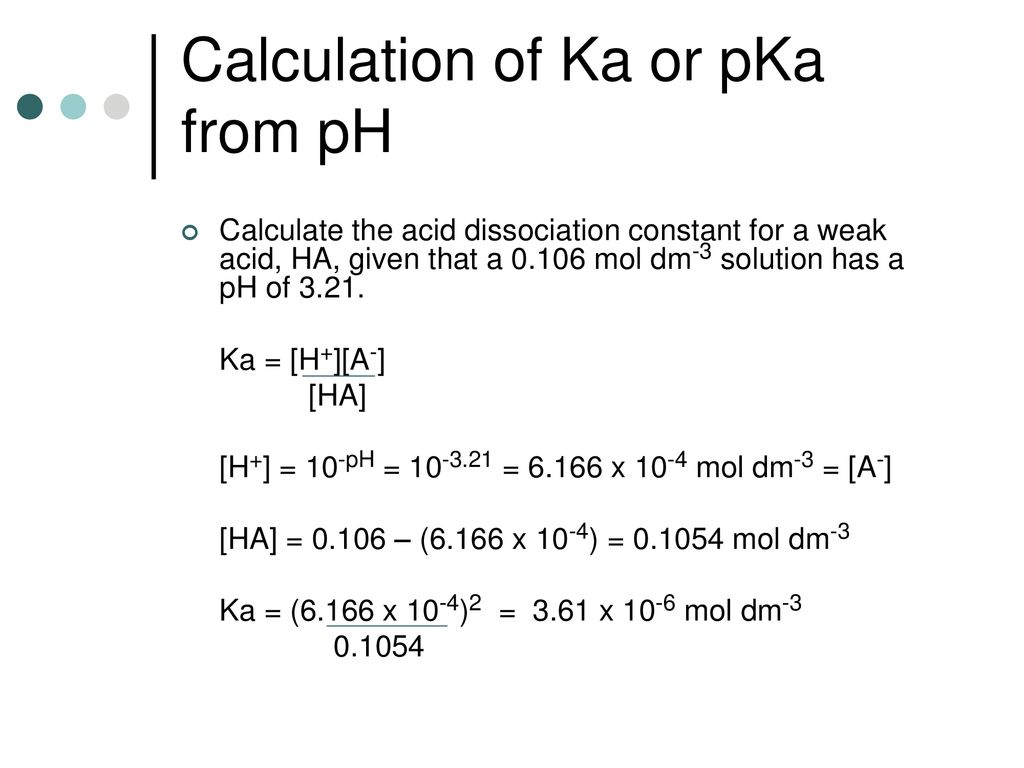
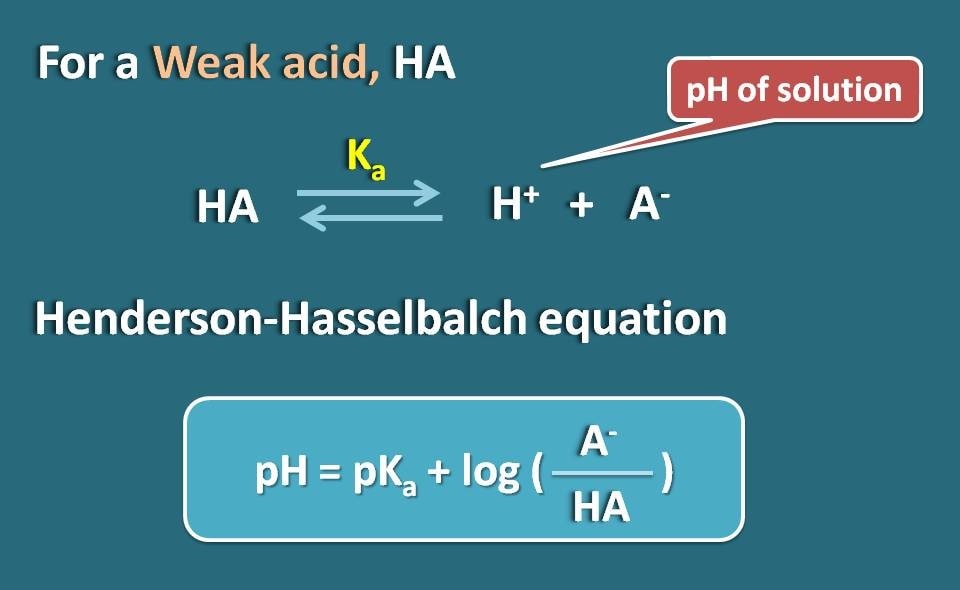





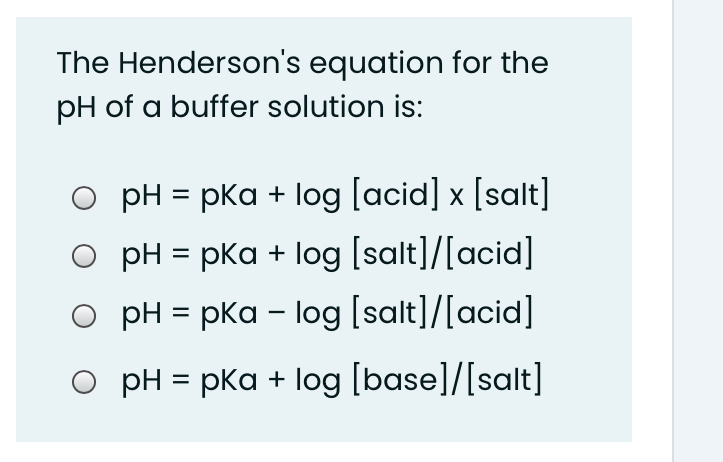

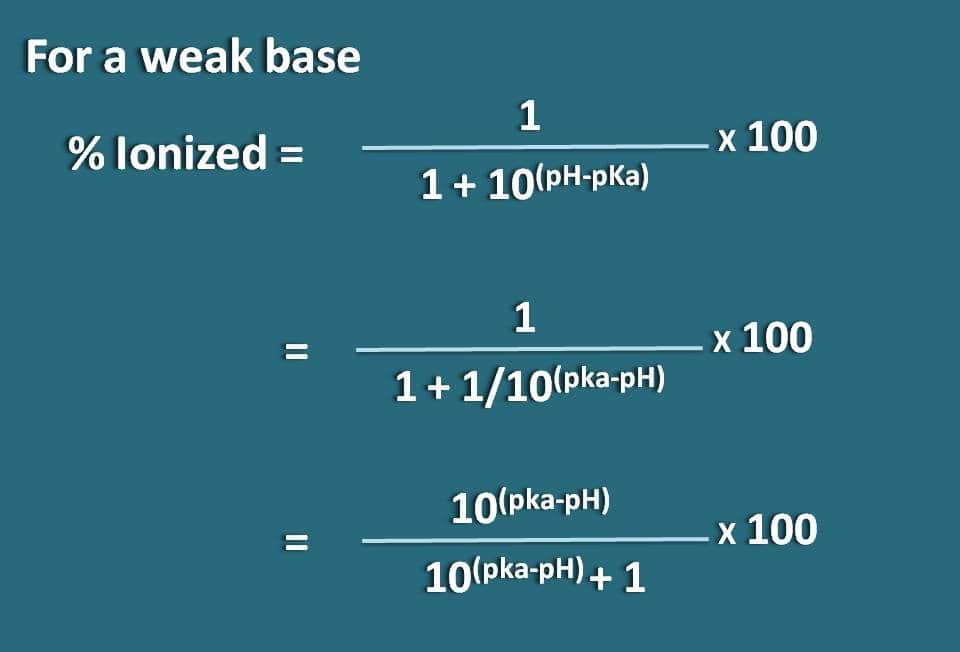
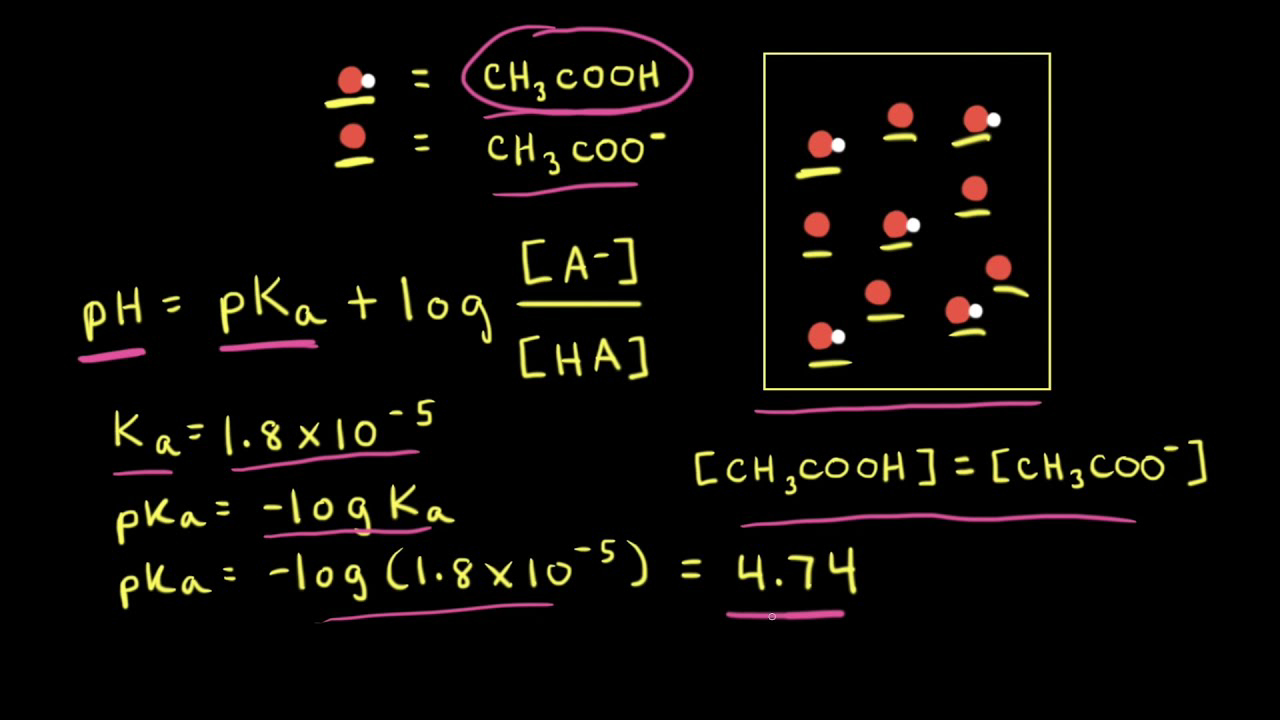



:max_bytes(150000):strip_icc()/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)